Hologenix: Infrared technology with potentially positive impact on diabetic patients
The diabetic community has always been a priority for Hologenix, creators of CELLIANT® infrared technology, so the company embarked on an initial study to test the hypothesis that the technology can positively impact diabetic patients with vascular impairment, now published in Journal of Textile Science & Engineering. Another study is underway as well with more research on the horizon.
According to statistics cited in the International Diabetes Federation Diabetes Atlas, 9th edition, globally, close to a half billion people are living with diabetes and that number is expected to increase by more than 50 percent in the next 25 years.
The introduction of the study in the Journal of Textile Science & Engineering also reports that diabetic patients frequently suffer from a combination of peripheral neuropathy and peripheral artery disease, which particularly affects their feet. It further states that it has been estimated that the lifetime risk for the development of foot ulcers in diabetic patients can be as high as 25 percent and that the risk of amputation is 10 to 20 times higher than in non-diabetic subjects.
The study was performed by Lawrence A. Lavery, D.P.M., M.P.H., a Professor in the Department of Plastic Surgery at UT Southwestern Medical Center. His clinic and research interests involve diabetic foot complications, infections and wound healing, and he participated in the conception, design, implementation and authorship of the Journal of Textile Science & Engineering study.
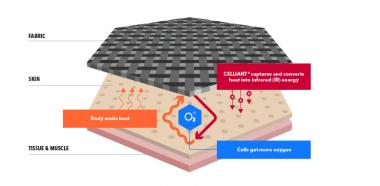
CELLIANT technology is a patented process for adding micron-sized thermo-responsive mineral particles to fibers, in this case polyethylene terephthalate (PET) fibers. The resulting CELLIANT yarns were woven into stockings and gloves containing either 82% CELLIANT polyester, 13% nylon and 5% spandex or for the placebo, 82% polyester with no CELLIANT, 13% nylon and 5% spandex. CELLIANT products absorb body heat and re-emit the energy back to the body as infrared energy, which is non-invasive and increases temporary blood flow and cell oxygenation levels in the body.
The objective of the study was to “evaluate changes in transcutaneous oxygen (TcPO2) and peripheral blood flow (laser Doppler, LD) in the hands and feet of diabetic patients with vascular impairment when CELLIANT gloves and stockings are worn.” While there was not a statistically significant result across all subjects, the study did show that some patients wearing CELLIANT stockings for 60 minutes had an increase of as much as 20% in tissue oxygenation and 30% in localized blood flow. According to the study’s conclusion, “the trends that were observed in favor of CELLIANT stockings suggest that a larger well-designed clinical trial should be undertaken and may provide evidence of clinical efficacy in treatment of the diabetic foot.”
The study also notes that “There have been no documented or observed side effects of wearing CELLIANT stockings, and they are relatively inexpensive compared to conventional pharmaceutical interventions.”
Hologenix has embarked on a more comprehensive trial, “Study to Evaluate CELLIANT Diabetic Medical Socks to Increase Tissue Oxygenation and Incidence of Complete Wound Closure in Diabetic Foot Wounds” – NCT04709419, which focuses on the impact of CELLIANT technology to potentially improve tissue oxygenation and wound healing outcomes.
“We are excited to explore whether future studies of infrared, with its most common biological effects of increased localized blood flow and cellular oxygenation, could result in a breakthrough in diabetic patients with vascular impairment,” said Seth Casden, Hologenix Co-founder and CEO. “We see a huge potential opportunity with this research for helping to fulfill our core mission of improving people’s health and well-being by potentially reducing the impact of diabetes, and we are actively seeking partners to expand our research efforts.”
Hologenix